Introduction to PANC-1 Xenograft Model
The PANC-1 cell line is one of the most widely used human pancreatic ductal adenocarcinoma (PDAC) models, originally derived from a pancreatic carcinoma in a 56-year-old male patient. These cells are characterized by their epithelial morphology, moderate differentiation, and presence of the commonly mutated KRAS gene (specifically, KRAS G12D), which is a hallmark of PDAC oncogenesis. Because of this, the PANC-1 xenograft model serves as an excellent system for studying KRAS-driven pancreatic cancer biology, tumor progression, and therapeutic response.
When implanted into immunocompromised mice, PANC-1 cells reliably generate tumors that closely mimic human pancreatic cancer histopathology, including dense stromal reaction and heterogeneous tumor cell populations. This makes the model especially valuable for preclinical drug evaluation and mechanistic studies of tumor microenvironment interactions.
Tumor Growth Characteristics and Histology
PANC-1 xenografts typically develop palpable tumors within 10 to 14 days post-implantation, exhibiting moderate to rapid growth rates. The tumors often reach volumes sufficient for longitudinal studies within 3 to 4 weeks. Histologically, PANC-1 tumors present as moderately differentiated adenocarcinomas with duct-like structures and an abundant desmoplastic stroma, recapitulating the complex tumor-stroma interplay observed in patient tumors.
The stroma consists of activated pancreatic stellate cells, fibroblasts, and infiltrating immune cells, contributing to extracellular matrix deposition and signaling crosstalk that influence tumor growth, invasion, and therapeutic resistance. This dynamic microenvironmental component is a critical feature of the PANC-1 model, supporting investigations into stroma-targeted therapies and tumor-host interactions.
Applications in Therapeutic Testing
Due to the presence of KRAS mutations, the PANC-1 xenograft is a prime model for evaluating novel therapies aimed at KRAS signaling pathways, as well as downstream effectors such as the MAPK/ERK and PI3K/AKT cascades. It has been widely used to test chemotherapeutic agents including gemcitabine, 5-fluorouracil, and novel targeted inhibitors.
Furthermore, the PANC-1 model is increasingly utilized in immunotherapy research, especially in humanized mouse models that allow partial reconstitution of the immune system. This enables the study of immune checkpoint inhibitors, adoptive cell transfer, and tumor vaccine strategies within a relevant pancreatic cancer context.
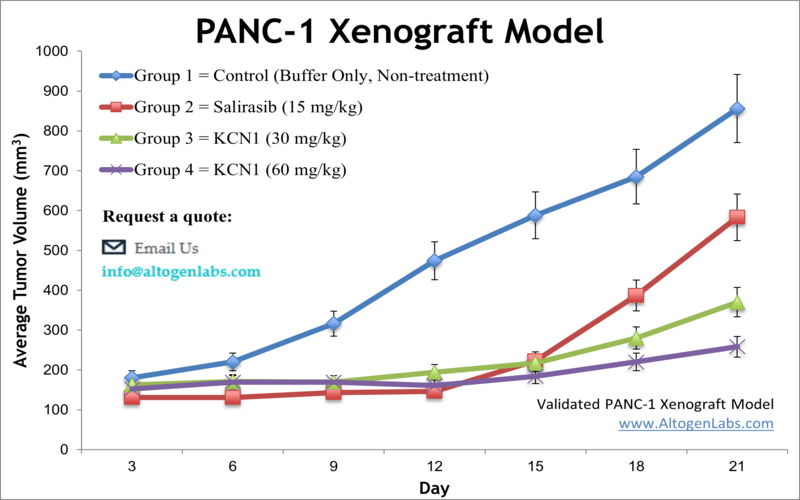
Altogen Labs PANC-1 Xenograft Model
The Altogen PANC-1 xenograft model is a well-established in vivo system for studying aggressive, poorly differentiated pancreatic ductal adenocarcinoma (PDAC). PANC-1 cells are derived from a human primary pancreatic tumor and are characterized by their mesenchymal morphology, slow but consistent proliferation, and a complex mutational profile that includes oncogenic KRAS (G12D), TP53 mutations, and CDKN2A inactivation. This genetic background mirrors the core molecular features of PDAC in patients, making the PANC-1 xenograft a relevant and reliable model for translational pancreatic cancer research.
When implanted subcutaneously into immunocompromised mice, PANC-1 cells give rise to solid, moderately vascularized tumors that demonstrate spindle-like cell morphology, a hallmark of epithelial-mesenchymal transition (EMT). These tumors typically develop within 3–4 weeks and exhibit reproducible growth patterns, enabling precise tumor burden monitoring over time. Despite their relatively slow doubling time in vitro, PANC-1 tumors in vivo show consistent tumor establishment and are highly amenable to longitudinal therapeutic studies.
The PANC-1 xenograft is extensively used to study drug resistance mechanisms, particularly resistance to gemcitabine and 5-FU, which are standard chemotherapeutics in PDAC treatment. The model is also valuable for assessing the role of key oncogenic pathways—including KRAS/MAPK, PI3K/AKT, and autophagy—in tumor maintenance and immune evasion. Its moderately desmoplastic microenvironment provides opportunities for evaluating tumor-stroma interactions and the efficacy of stroma-targeting agents.
Altogen Biosystems supports this model with a specialized PANC-1 in vivo transfection reagent that enables high-efficiency delivery of siRNA, shRNA, CRISPR/Cas9 components, and plasmid DNA directly into tumor tissue. This allows for dynamic manipulation of gene expression within the tumor microenvironment—critical for studying functional genomics, gene-drug interactions, and compensatory signaling pathways in vivo. The reagent is non-viral, low-toxicity, and designed for systemic or local administration, preserving tumor viability and morphology while achieving reliable gene knockdown or overexpression.
In summary, the Altogen PANC-1 xenograft model provides a versatile and genetically relevant platform for evaluating experimental therapeutics, identifying resistance pathways, and conducting mechanistic studies in a physiologically representative setting of pancreatic cancer.
Altogen PANC-1 Transfection Reagent: Enhancing In Vivo Genetic Manipulation
Altogen Biosystems provides a specialized transfection reagent optimized for efficient and low-toxicity delivery of nucleic acids into PANC-1 cells both in vitro and in vivo. This reagent is designed to form stable complexes with plasmid DNA, siRNA, miRNA, or CRISPR-Cas9 components, facilitating high-efficiency gene expression or silencing directly within xenograft tumors.
Its proprietary lipid-polymer formulation promotes rapid cellular uptake via endocytosis and effective endosomal escape, achieving transfection efficiencies exceeding 70% while maintaining cell viability above 90%. This enables functional genomic studies such as gene knockout, overexpression, or pathway modulation in a physiologically relevant tumor setting.
The reagent’s compatibility with physiological serum-containing media and stability for repeated dosing protocols make it ideal for longitudinal studies and therapeutic target validation in PANC-1 xenograft models.
Modeling Tumor Microenvironment and Metastasis
While subcutaneous implantation is commonly used for ease of monitoring tumor growth, orthotopic implantation of PANC-1 cells into the pancreas enables more clinically relevant studies of local invasion, metastasis, and tumor-stroma crosstalk. PANC-1 xenografts display invasive behavior and can metastasize to regional lymph nodes and distant organs, including liver and lungs, replicating critical aspects of pancreatic cancer dissemination.
This metastatic potential supports research into the molecular mechanisms underlying tumor progression and the development of anti-metastatic therapies. Additionally, the model facilitates investigation of tumor-induced immunosuppression and angiogenesis, important factors that contribute to therapeutic resistance.
Research Applications and Benefits
The PANC-1 xenograft model is instrumental in studies of oncogenic signaling, drug resistance mechanisms, tumor microenvironment interactions, and immunomodulation. Its genetic background and biological behavior closely parallel many human PDAC cases, enhancing translational relevance.
The Altogen PANC-1 Transfection Reagent significantly expands experimental versatility by allowing precise genetic manipulation within the tumor in vivo. This enables functional studies of candidate genes, validation of therapeutic targets, and screening of gene-environment interactions within a complex tumor milieu.
Researchers employing this model gain access to a robust system combining clinically relevant tumor biology with advanced molecular tools for comprehensive pancreatic cancer investigation.
Request a Quote
For detailed information, pricing, and availability of the Altogen PANC-1 Transfection Reagent designed for high-efficiency in vivo and in vitro gene delivery, please visit.
