Introduction to MIA PaCa-2 Xenograft Model
The MIA PaCa-2 cell line is one of the most widely used human pancreatic ductal adenocarcinoma (PDAC) models, originally established from a primary pancreatic carcinoma. This cell line exhibits a highly aggressive phenotype characterized by rapid proliferation, strong tumorigenicity, and a propensity for invasion and metastasis, closely mirroring the clinical course of advanced pancreatic cancer. MIA PaCa-2 cells harbor key mutations such as KRAS and TP53, which drive oncogenic signaling pathways fundamental to pancreatic tumor biology.
When implanted as xenografts in immunodeficient mice, MIA PaCa-2 cells form tumors that recapitulate the histopathological and molecular hallmarks of human PDAC, making them invaluable for preclinical therapeutic testing, tumor biology studies, and exploration of drug resistance mechanisms. The ability to grow these tumors both subcutaneously and orthotopically into the pancreas provides flexibility in experimental design, enabling evaluation of both primary tumor growth and metastatic dissemination.
Tumor Growth Characteristics and Model Stability
MIA PaCa-2 xenografts are characterized by fast and robust tumor formation, typically reaching measurable size within 10 to 14 days after implantation. Subcutaneous tumors provide an accessible site for monitoring growth kinetics, while orthotopic models more accurately replicate the pancreatic tumor microenvironment, including stromal interactions, desmoplasia, and local invasion.
Histologically, MIA PaCa-2 tumors display poorly differentiated adenocarcinoma features with a dense stromal matrix and abundant fibroblast infiltration. This microenvironment supports studies on tumor-stroma crosstalk, a critical factor in PDAC progression and therapy resistance. Furthermore, these xenografts often show variable vascularization, allowing investigation of anti-angiogenic treatments and drug delivery challenges inherent to pancreatic tumors.
Applications in Drug Development and Resistance Research
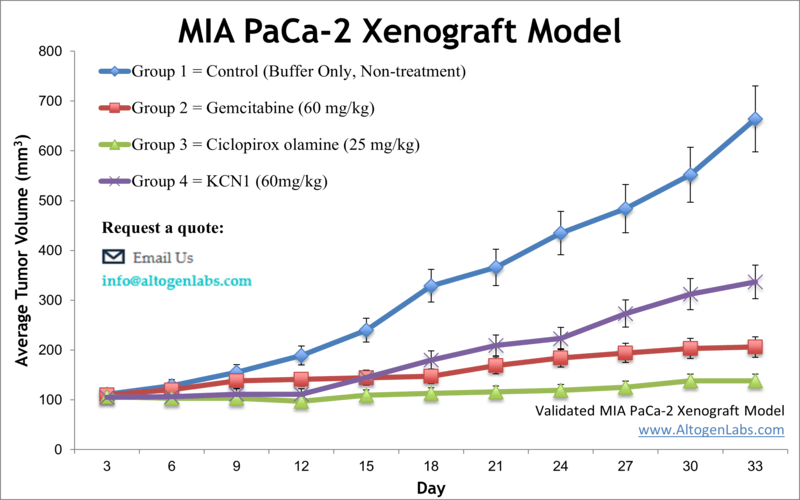
The aggressive nature and well-characterized molecular profile of MIA PaCa-2 xenografts make them a preferred model for testing novel chemotherapeutic agents, targeted therapies, and combination regimens. These tumors respond variably to standard treatments like gemcitabine, reflecting the clinical heterogeneity seen in PDAC patients. This variability enables the study of mechanisms underlying chemoresistance and the identification of biomarkers predictive of therapeutic response.
MIA PaCa-2 xenografts have been instrumental in evaluating inhibitors targeting key pathways such as MAPK/ERK, PI3K/AKT/mTOR, and Hedgehog, as well as agents modulating the tumor microenvironment. The model supports investigation into novel drug delivery systems, including nanoparticle-mediated therapies and stroma-modifying approaches aimed at enhancing drug penetration and efficacy.
Altogen Labs MIA PaCa-2 Xenograft Model
The Altogen MIA PaCa-2 xenograft model is a widely adopted and aggressive in vivo system for studying poorly differentiated pancreatic ductal adenocarcinoma (PDAC). Derived from a primary pancreatic tumor, MIA PaCa-2 cells exhibit mesenchymal-like morphology, rapid proliferation, and hallmark oncogenic mutations, including KRAS (G12C), TP53, and CDKN2A deletions, closely mirroring the molecular landscape of advanced human pancreatic cancers. When implanted into immunocompromised mice, MIA PaCa-2 cells reliably form fast-growing, undifferentiated tumors with high cellularity and minimal stromal involvement. This makes the model particularly well-suited for investigating tumor-intrinsic signaling, oncogene addiction, and chemoresistance mechanisms in a streamlined, epithelial-depleted tumor environment.
The MIA PaCa-2 xenograft is frequently utilized in evaluating cytotoxic drugs, kinase inhibitors, and epigenetic modulators due to its aggressive biology and reproducible growth patterns. It is also a valuable system for studying the metabolic reprogramming typical of PDAC, such as altered glucose uptake and autophagy dependence. The model’s high tumor burden and short latency period allow for rapid in vivo screening of therapeutic agents.
Altogen Biosystems enhances the utility of this model with its in vivo-optimized MIA PaCa-2 Transfection Reagent, enabling efficient delivery of plasmid DNA, siRNA, miRNA, or CRISPR/Cas9 components directly into tumor tissue. This facilitates powerful gene modulation studies within the xenograft context, such as gene knockdown of drug resistance mediators, validation of synthetic lethal targets, and exploration of signaling pathways that drive tumor maintenance. The MIA PaCa-2 xenograft model, combined with Altogen’s advanced delivery technologies, offers a high-throughput and biologically relevant system for translational pancreatic cancer research.
In Vivo Gene Manipulation Using Altogen Transfection Reagent
Altogen Biosystems offers a specialized MIA PaCa-2 Transfection Reagent optimized for efficient in vivo gene delivery within xenograft tumors. This reagent is formulated to facilitate high transfection efficiency with minimal cytotoxicity, allowing researchers to introduce plasmid DNA, siRNA, miRNA, or CRISPR/Cas9 constructs directly into tumor tissue.
The reagent’s design promotes effective nucleic acid complexation and cellular uptake via endocytosis, ensuring robust gene expression or knockdown within the tumor microenvironment. Its compatibility with complete growth media and stability under physiological conditions enable repeated dosing and longitudinal studies of gene function in vivo.
Utilizing Altogen’s reagent, researchers can perform functional genomics experiments to dissect oncogenic signaling, validate therapeutic targets, or explore resistance mechanisms in the complex in vivo context of MIA PaCa-2 tumors. This capability significantly enhances the translational relevance of xenograft studies.
Modeling Metastasis and Tumor Microenvironment Interactions
Beyond primary tumor growth, MIA PaCa-2 xenografts can be used to investigate metastatic progression, especially when implanted orthotopically. These models exhibit local invasion and can seed metastases to organs such as the liver and lungs, enabling comprehensive studies of metastatic biology.
The tumor microenvironment in MIA PaCa-2 xenografts comprises not only cancer cells but also activated fibroblasts, immune cells, and extracellular matrix components. This complexity supports research into stromal remodeling, immune evasion, and angiogenesis, which are pivotal in pancreatic cancer progression and therapeutic resistance. Studies combining genetic manipulation with microenvironment modulation are feasible and highly informative using this model.
Research Utility
The MIA PaCa-2 xenograft model stands as a powerful and versatile system for pancreatic cancer research. Its aggressive growth, metastatic potential, and clinically relevant molecular characteristics offer a robust platform for preclinical drug evaluation, mechanistic studies, and biomarker discovery. Integration with Altogen Biosystems’ in vivo transfection reagent expands experimental possibilities by enabling precise genetic interventions within tumor tissue.
For researchers aiming to leverage the MIA PaCa-2 model for therapeutic development or functional genomics, Altogen’s transfection reagent offers a reliable and effective tool to enhance the biological insights gained from these in vivo studies.
Request a Quote
To request pricing, availability, or more information about the MIA PaCa-2 Transfection Reagent, please visit the link. Altogen Biosystems provides dedicated support to tailor solutions for your specific research needs.
