Introduction to Capan-1 Xenograft Models
The Capan-1 cell line is a well-established human pancreatic adenocarcinoma line originally derived from a liver metastasis of a pancreatic ductal adenocarcinoma (PDAC) patient. Capan-1 cells retain many genetic and phenotypic features characteristic of moderately differentiated pancreatic tumors, including key mutations in the KRAS and TP53 genes. Their epithelial morphology and ability to form glandular-like structures in vitro make them an important model for studying pancreatic tumor biology and drug response, particularly in metastatic contexts.
Capan-1 xenograft models are typically generated in immunocompromised mice, such as nude or NOD/SCID strains, through subcutaneous or orthotopic implantation. These models reliably produce tumors that mimic the histopathological and molecular characteristics of human PDAC, making them invaluable for preclinical drug evaluation, tumor microenvironment research, and metastatic progression studies. Their moderate growth rate and consistent tumor take rate allow reproducible assessment of therapeutic interventions.
Tumorigenic Characteristics and Growth Dynamics
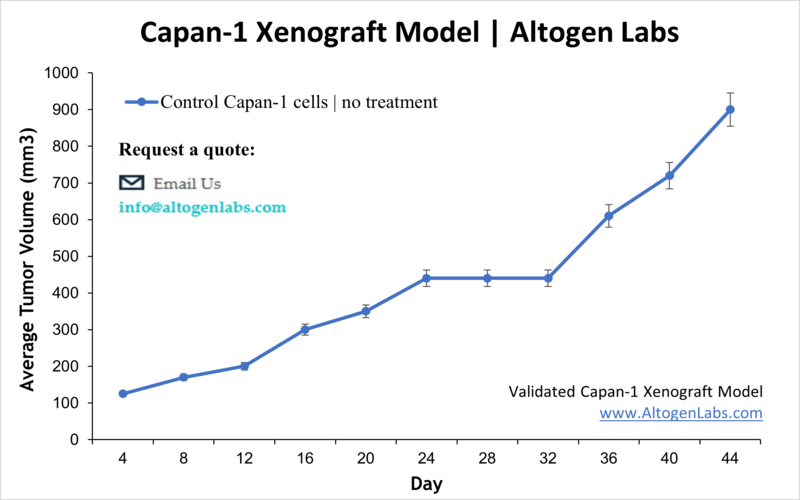
Capan-1 xenografts grow steadily, with palpable tumors emerging approximately 2 to 3 weeks post-implantation when injected subcutaneously. Orthotopic implantation into the pancreas further enhances physiological relevance by recreating native tumor-stromal interactions, desmoplasia, and vascularization patterns. Compared to other pancreatic cancer xenograft models, Capan-1 tumors demonstrate moderate invasiveness but pronounced capacity for liver metastasis, which is essential for studying metastatic mechanisms and therapeutic resistance.
Histologically, Capan-1 xenografts preserve the glandular architecture of the original tumor, displaying epithelial cell polarity and mucin production. This characteristic provides a platform to investigate the tumor-stroma interplay and the influence of extracellular matrix components on tumor progression. Moreover, the tumor microenvironment in these xenografts contains abundant fibroblasts and immune cells, enabling evaluation of stroma-targeted therapies and immunomodulatory agents.
Utility in Therapeutic Testing and Drug Resistance
Capan-1 xenografts have been extensively employed in preclinical evaluation of chemotherapeutic agents, targeted therapies, and novel drug delivery systems. Their intermediate sensitivity to gemcitabine and other nucleoside analogues models clinically relevant partial resistance scenarios observed in PDAC patients. This makes Capan-1 a suitable platform for testing combination therapies aimed at overcoming drug resistance.
The model is also widely used for testing inhibitors of key oncogenic pathways such as MAPK, PI3K/AKT, and Hedgehog signaling. Due to the preservation of intact stromal components, Capan-1 xenografts are ideal for studying the effects of tumor microenvironment modulation on drug response, including anti-fibrotic and anti-angiogenic agents.
Altogen Labs Capan-1 Xenograft Model
The Altogen Capan-1 xenograft model provides a highly relevant platform for studying moderately differentiated pancreatic ductal adenocarcinoma (PDAC) with prominent epithelial characteristics. Originating from a liver metastasis of a human pancreatic cancer patient, Capan-1 cells are notable for their well-formed glandular structures, expression of epithelial markers such as E-cadherin and cytokeratins, and secretion of high levels of carcinoembryonic antigen (CEA), making them suitable for studies involving tumor marker tracking and differentiation status. When implanted into immunodeficient mice, Capan-1 cells form slow-growing, well-delineated tumors that maintain histological features of ductal origin and often exhibit a mucinous component. This model is ideal for evaluating differentiation-related pathways, epithelial integrity, and therapeutic responses targeting glandular tumor architecture. The Altogen Capan-1 xenograft system is frequently used in preclinical testing of chemotherapeutics and targeted biologics, particularly those affecting cell-cell adhesion, mucin production, and epithelial signaling. Additionally, with Altogen’s specialized in vivo transfection reagents, researchers can genetically manipulate Capan-1 tumors in situ, facilitating studies in gene silencing, overexpression, or CRISPR-mediated genome editing under biologically relevant tumor conditions.
In Vivo Gene Delivery with Altogen Transfection Reagents
Altogen Biosystems provides a specialized Capan-1 transfection reagent optimized for efficient genetic manipulation in vivo. This reagent is designed for direct intratumoral delivery and supports transfection of siRNA, miRNA, plasmid DNA, and CRISPR/Cas9 components within Capan-1 xenografts. The formulation promotes high transfection efficiency and robust gene modulation with minimal cytotoxicity or disruption of the tumor microenvironment.
Using the Altogen reagent, researchers can perform functional genomics studies such as gene knockdown, overexpression, and genome editing directly within the tumor tissue. This facilitates target validation, exploration of resistance mechanisms, and assessment of gene function in the complex in vivo setting. The reagent’s compatibility with complete growth media and ease of use make it suitable for repeated dosing regimens and long-term studies.
Modeling Metastasis and Tumor Microenvironment
The Capan-1 xenograft model is especially valuable for studying metastatic progression due to its propensity for liver dissemination. Orthotopic implantation into the pancreas enhances metastasis rates, allowing detailed analysis of the metastatic cascade, including invasion, intravasation, and colonization of secondary sites. Fluorescent or bioluminescent labeling of Capan-1 cells further enables real-time imaging and longitudinal tracking of tumor spread.
Beyond metastasis, the model captures the dense desmoplastic stroma characteristic of human PDAC. This enables evaluation of stromal-targeted treatments and immunotherapy strategies within a relevant microenvironment. Studies involving co-transplantation of immune or stromal cells, or modulation of the extracellular matrix, are feasible using this system.
Research Applications
The Capan-1 xenograft model serves as a robust and clinically relevant platform for pancreatic cancer research. Its moderate tumorigenicity, metastatic capability, and preservation of tumor-stroma interactions make it a valuable tool for drug discovery, mechanistic studies, and translational research. Coupled with Altogen Biosystems’ in vivo transfection technologies, this model allows sophisticated genetic manipulation directly in tumor tissue, expanding the experimental possibilities in functional genomics and therapeutic validation.
Request a Quote
If you would like to inquire about pricing, availability, or bulk orders for the Capan-1 Transfection Reagent, or need technical support, please follow the link to request a personalized quote from Altogen Biosystems. Our team offers prompt responses and customized service to meet your research needs.
