Introduction to BxPC-3 Xenograft Model
The BxPC-3 cell line is a well-established human pancreatic ductal adenocarcinoma (PDAC) model derived from a primary pancreatic tumor. Unlike many pancreatic cancer lines that harbor KRAS mutations, BxPC-3 cells are KRAS wild-type, providing a valuable contrast for studying KRAS-independent pathways and tumor biology. These cells are moderately differentiated and display epithelial morphology, making them relevant for investigations into PDAC differentiation status and tumor progression.
When implanted as xenografts in immunodeficient mice, BxPC-3 cells form tumors that replicate several key features of human pancreatic cancer, including glandular architecture and desmoplastic stromal reaction. This model is widely used for drug efficacy testing, tumor microenvironment studies, and biomarker discovery.
Tumor Growth and Histopathology
BxPC-3 xenografts typically establish palpable tumors within 14 to 21 days post-implantation, with growth rates somewhat slower than highly aggressive lines such as MIA PaCa-2, reflecting their less aggressive phenotype. The tumors exhibit well-formed ductal structures and mucin production, replicating glandular features observed in patient tumors.
Histological analysis shows a dense stromal matrix with activated fibroblasts and immune infiltrates, recapitulating the desmoplastic environment characteristic of PDAC. This makes the BxPC-3 model particularly useful for exploring tumor-stroma interactions, extracellular matrix remodeling, and the impact of stroma on therapeutic response.
Applications in Therapeutic Development
Due to the absence of KRAS mutations, BxPC-3 xenografts provide a complementary model to KRAS-mutant PDAC lines, enabling evaluation of targeted therapies that operate through KRAS-independent mechanisms. This includes agents targeting growth factor receptors, cell cycle regulators, and apoptotic pathways.
BxPC-3 tumors respond to standard chemotherapeutics such as gemcitabine and nab-paclitaxel, though with varying sensitivity, allowing for studies into mechanisms of drug resistance and combination strategies. The model is also suited for evaluating novel immunotherapies, given the presence of an immune-infiltrated microenvironment that can be partially reconstituted in humanized mouse models.
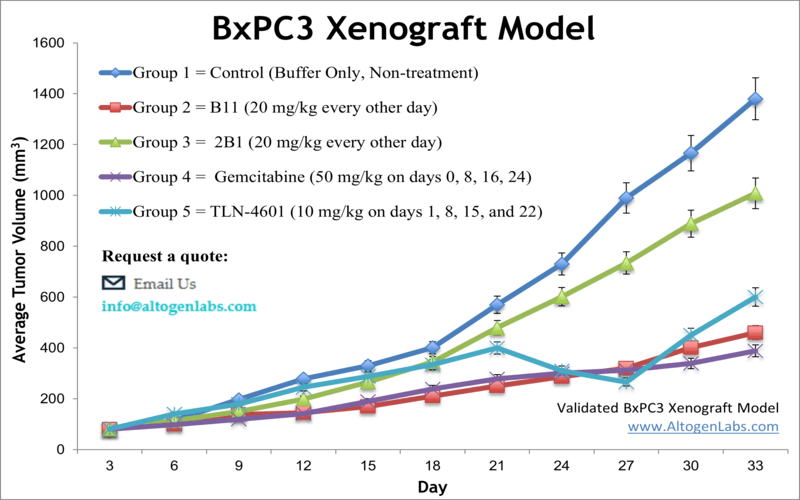
Altogen Labs BxPC-3 Xenograft Model
The Altogen BxPC-3 xenograft model is a clinically relevant in vivo system for studying moderately differentiated, KRAS-wildtype pancreatic ductal adenocarcinoma (PDAC). BxPC-3 cells are unique among commonly used pancreatic cancer cell lines due to their intact wild-type KRAS gene and relatively well-differentiated phenotype, which includes organized epithelial morphology and expression of tight junction proteins and mucin markers. These properties make the BxPC-3 xenograft especially valuable for research focused on non-KRAS-driven oncogenesis, tumor-stroma interactions, and drug responses in genetically distinct subsets of PDAC.
When injected subcutaneously into immunodeficient mice, BxPC-3 cells generate highly vascularized tumors with well-formed glandular architecture and a moderate proliferation rate. Tumors derived from BxPC-3 cells closely mirror the epithelial features seen in differentiated pancreatic tumors, including strong E-cadherin expression and structured cell junctions. This allows for high-fidelity modeling of epithelial integrity, differentiation signaling, and tumor microenvironment crosstalk.
The BxPC-3 xenograft model is widely used in preclinical oncology for evaluating the efficacy of EGFR inhibitors, anti-angiogenic therapies, and novel compounds targeting the PI3K/AKT/mTOR pathway, which is commonly activated in KRAS-independent tumors. Its consistent tumor growth kinetics and clear histopathology also support pharmacokinetic and pharmacodynamic analyses of drug candidates.
Altogen Biosystems offers a tailored in vivo transfection reagent for BxPC-3 xenografts, enabling efficient delivery of genetic material directly into tumors. This reagent facilitates the modulation of gene expression in vivo—critical for validating therapeutic targets, conducting pathway interference studies, and modeling resistance mechanisms. Researchers benefit from the ability to perform non-viral gene editing and RNA interference within the tumor context without compromising tissue integrity or inducing immune responses.
Overall, the Altogen BxPC-3 xenograft model provides a robust, reproducible platform for investigating KRAS-wildtype pancreatic cancer biology and for testing targeted therapies in a differentiation-retaining PDAC setting.
Genetic Manipulation Using Altogen Transfection Reagent
Altogen Biosystems offers a specialized BxPC-3 Transfection Reagent formulated to achieve high-efficiency gene delivery in vivo with minimal cytotoxicity. This reagent enables direct introduction of plasmid DNA, siRNA, miRNA, or CRISPR-Cas9 constructs into BxPC-3 xenograft tumors, facilitating functional genomics studies within the tumor microenvironment.
The reagent’s optimized formulation promotes stable nucleic acid complexation and efficient cellular uptake via endocytosis, ensuring robust gene expression or knockdown with minimal off-target effects. Its compatibility with physiological media and stability in vivo allows repeated dosing and longitudinal studies, critical for modeling dynamic tumor processes.
This capability expands experimental options, permitting the interrogation of gene function, signaling pathways, and therapeutic target validation directly in BxPC-3 tumors, thus enhancing translational relevance.
Modeling Tumor Microenvironment and Metastasis
While BxPC-3 xenografts primarily grow subcutaneously for ease of monitoring, orthotopic implantation into the pancreas allows investigation of local tumor-stroma interactions and metastatic spread. The model supports studies on desmoplasia, angiogenesis, and immune cell infiltration, which are key factors influencing PDAC progression and therapeutic response.
BxPC-3 tumors exhibit limited spontaneous metastasis, making them useful for examining early-stage tumor growth and microenvironmental influences rather than extensive metastatic dissemination. Combining genetic manipulation with microenvironment modulation enables comprehensive analysis of factors driving tumor development and resistance.
Research Value
The BxPC-3 xenograft model is an essential tool in pancreatic cancer research, especially for studying KRAS wild-type tumors and their unique biology. Its moderate growth, glandular differentiation, and rich stromal content provide a robust platform for drug testing, tumor microenvironment exploration, and biomarker identification.
Utilizing Altogen Biosystems’ in vivo transfection reagent tailored for BxPC-3 cells enhances the capacity to perform detailed functional studies, accelerating translational research and therapeutic innovation.
Request a Quote
For inquiries about pricing, availability, or detailed technical support for the BxPC-3 Transfection Reagent, please visit the link. Altogen Biosystems is committed to supporting your research with high-quality, reliable products.
