Introduction to AsPC-1 Xenograft Models
The AsPC-1 cell line is a human pancreatic adenocarcinoma cell line derived from ascites fluid of a patient with metastatic pancreatic cancer. It is one of the most aggressive and widely used cell lines in preclinical studies due to its high tumorigenicity and capability to form xenografts with robust metastatic potential. AsPC-1 cells harbor key genetic mutations relevant to pancreatic cancer pathogenesis, including KRAS, TP53, and SMAD4, and exhibit properties of poorly differentiated adenocarcinoma. These cells are characterized by rapid proliferation, epithelial morphology, and secretion of mucin and other tumor markers, making them highly representative of advanced-stage PDAC.
AsPC-1 xenograft models are typically established in immunodeficient mice, including athymic nude or NOD/SCID strains, through subcutaneous or orthotopic implantation. These models are crucial for studying tumor progression, metastatic dissemination, and therapeutic resistance, as AsPC-1 cells readily metastasize to the liver, lungs, and peritoneum. Given their aggressive nature and reproducibility, AsPC-1 xenografts are valuable tools for evaluating novel chemotherapeutics, targeted inhibitors, and gene therapies.
Tumorigenic and Metastatic Behavior
AsPC-1 xenografts demonstrate a high degree of invasiveness and metastatic behavior compared to other pancreatic cancer models. Subcutaneous implantation results in palpable tumors within 10–14 days post-injection, with consistent growth kinetics. However, orthotopic implantation into the pancreas more accurately recapitulates the tumor microenvironment and results in spontaneous metastasis, thus enabling studies on tumor spread and site-specific colonization. AsPC-1 tumors are often poorly encapsulated, with extensive stromal interaction and vascular infiltration, providing a more clinically relevant model for tumor-host interactions and desmoplastic responses.
Utility in Drug Screening and Resistance Studies
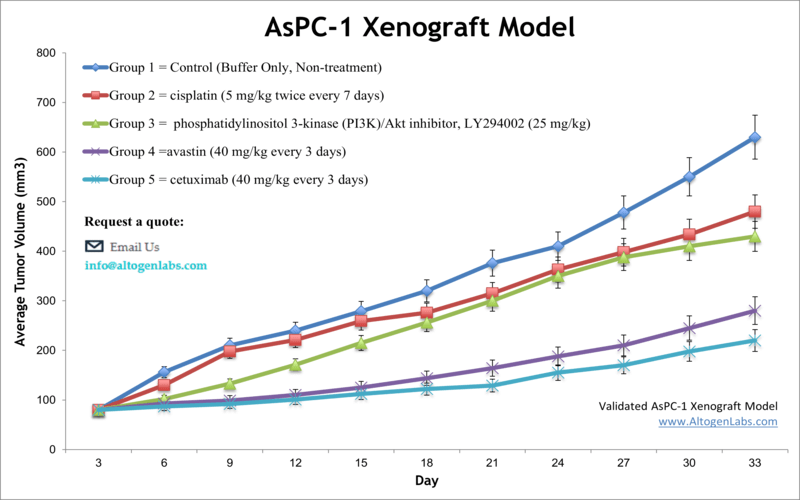
AsPC-1 xenografts are widely used in preclinical drug screening due to their robust growth and reproducibility. These models respond variably to standard-of-care treatments like gemcitabine and show pronounced resistance to monotherapies targeting EGFR or MEK pathways. This makes them ideal for studying mechanisms of drug resistance and testing combination therapies. The model is also used for evaluating anti-angiogenic agents, as AsPC-1 tumors are moderately vascularized and express VEGF and other angiogenic factors.
Furthermore, the AsPC-1 model supports real-time imaging and longitudinal monitoring when labeled with luciferase or fluorescent proteins. This feature is particularly advantageous for high-throughput drug testing and kinetic analysis of tumor response.
Altogen Labs AsPC-1 Xenograft Model
The Altogen AsPC-1 xenograft model is a robust and well-characterized in vivo system for studying advanced pancreatic ductal adenocarcinoma (PDAC). Derived from a metastatic ascites sample of a human pancreatic cancer patient, AsPC-1 cells exhibit high tumorigenicity, aggressive growth kinetics, and strong metastatic potential, particularly to liver and lymphatic tissues. These features make the AsPC-1 xenograft an ideal model for investigating late-stage disease progression, metastasis, and treatment resistance. When injected subcutaneously or orthotopically into immunocompromised mice, AsPC-1 cells rapidly form tumors that recapitulate key histopathological features of human PDAC, including desmoplasia, vascularization, and epithelial-mesenchymal transition (EMT) signatures. Altogen Biosystems supports this model with optimized reagents and protocols for in vivo gene modulation, enabling researchers to test the efficacy of novel therapeutics, evaluate oncogenic signaling pathways, and conduct CRISPR-based functional genomics in a metastatic pancreatic cancer context.
In Vivo Gene Manipulation Using Altogen Transfection Kits
Altogen Biosystems provides a dedicated AsPC-1 in vivo transfection reagent designed for direct delivery of genetic material into tumors formed from this cell line. The Altogen AsPC-1 transfection reagent is optimized for intratumoral injection and supports high-efficiency delivery of siRNA, miRNA, plasmid DNA, and CRISPR components. It enables gene knockdown or overexpression in the tumor microenvironment, supporting functional genomics studies, target validation, and exploration of oncogenic signaling networks.
The nanoparticle-based formulation ensures deep tissue penetration and minimal cytotoxicity. Transfection efficiency typically exceeds 70% for plasmid DNA and up to 90% for siRNA with preserved tumor viability. This makes it a valuable tool for in vivo gene modulation and for creating genetically engineered xenograft models.
Integration with Orthotopic and Systemic Models
The use of orthotopic AsPC-1 xenografts enhances the model’s relevance by enabling the assessment of tumor growth within the native pancreatic milieu. These models are suitable for evaluating surgical interventions, radiation therapies, and local drug delivery systems. Systemic dissemination can be studied using tail vein or intracardiac injection of AsPC-1 cells, modeling hematogenous metastasis and secondary organ colonization.
In vivo transfection can be integrated with these advanced models by tailoring the Altogen reagent’s administration route—either direct injection into the pancreatic mass or systemic delivery through intravenous routes—to suit experimental needs. This adaptability makes the Altogen transfection system uniquely suited for complex in vivo studies.
Request a Quote
If you are interested in using the AsPC-1 Transfection Reagent for your xenograft studies or would like to inquire about pricing, bulk orders, or technical specifications, Altogen Biosystems offers personalized assistance and support. Please follow the link to request a quote or contact our scientific support team. Fast response times and custom order fulfillment are available to meet research project timelines and scale.
